Here’s the protocol for preparing and running 30 percent SDS-PAGE gels:
1. Retrieve the SDS gels as these are the ones we’ll be using for this procedure.
2. Prepare your extracted samples by gathering the following items: a tray with crushed ice, pipettor, 96-well plate, extraction buffer, sample buffer, and the protein extracts (the samples that have been extracted). Thaw the samples and ensure they are fully defrosted. Use a vortex to thoroughly mix the samples.
3. Take 20 microtubes containing protein extracts and place them in a centrifuge. Spin the centrifuge for five minutes to separate any debris.
4. Label the 96-well plate accordingly, designating ten wells for rice and ten for maize samples.
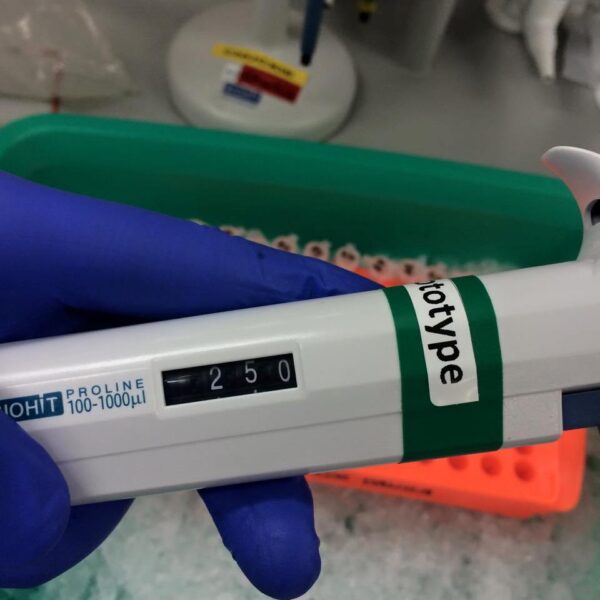
5. With the pipettor set to 50 µL, add the sample buffer to each well.
6. Reset the pipettor to 35 µL and add the extraction buffer to the designated wells.
7. Reset the pipettor again to 15 µL and add the protein extracts to each well, where the sample and extract buffers have been placed.
8. Heat the 96-well plate at 95°C for five minutes, then immediately place it on ice to ensure that the proteins are properly denatured and ready for use.
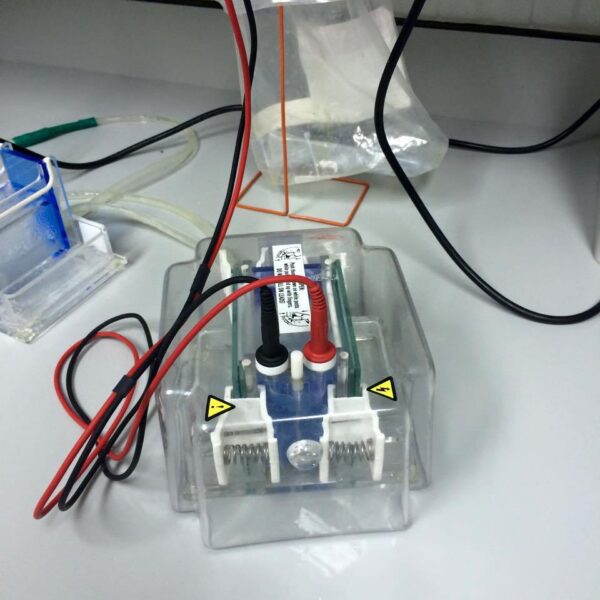
9. Prepare a 10 percent acrylamide gel with ten wells for loading. Insert this gel into the SDS gel machine and pour the running buffer on top.
10. Adjust the pipettor to 10 µL for loading the prepared samples. The loading format can be set as needed based on your samples. For example: M1, M2, M3, M4, M5, R1, R2, R3, R4, R5, and so on.
11. Once all samples are loaded, set the machine to run at 160 volts for 80 minutes to perform electrophoresis.
12. Once the SDS gels containing protein samples are ready, transfer them to individual containers. Add gel fixer and place the containers on a shaker for 30 minutes. Then, immerse the gels in a gel stain for 20 minutes, followed by a gel de-stain for 45 minutes. Finally, store the gels for at least one hour.
13. Your gels are now ready for viewing using a Gel Doc or similar equipment.